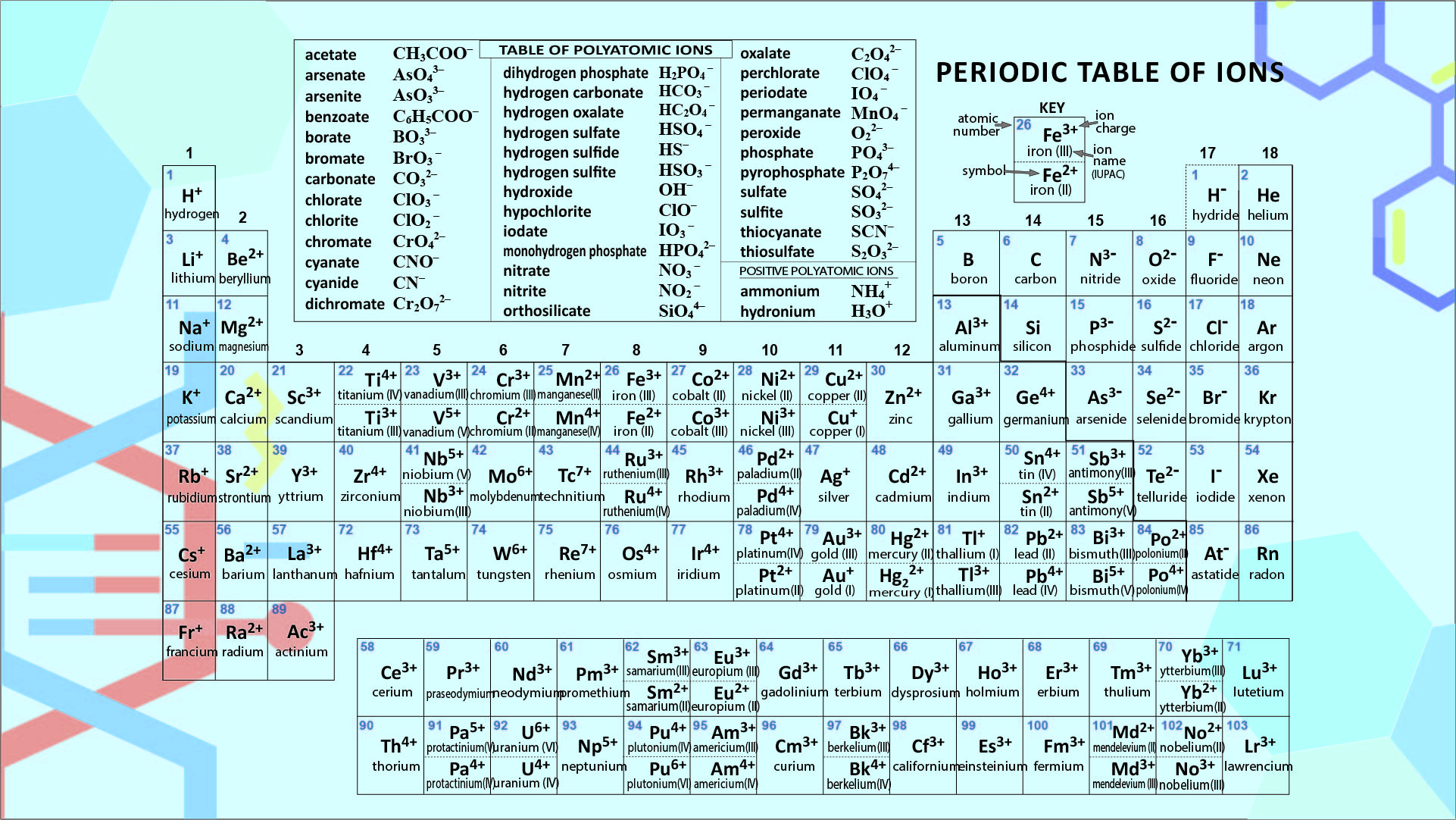
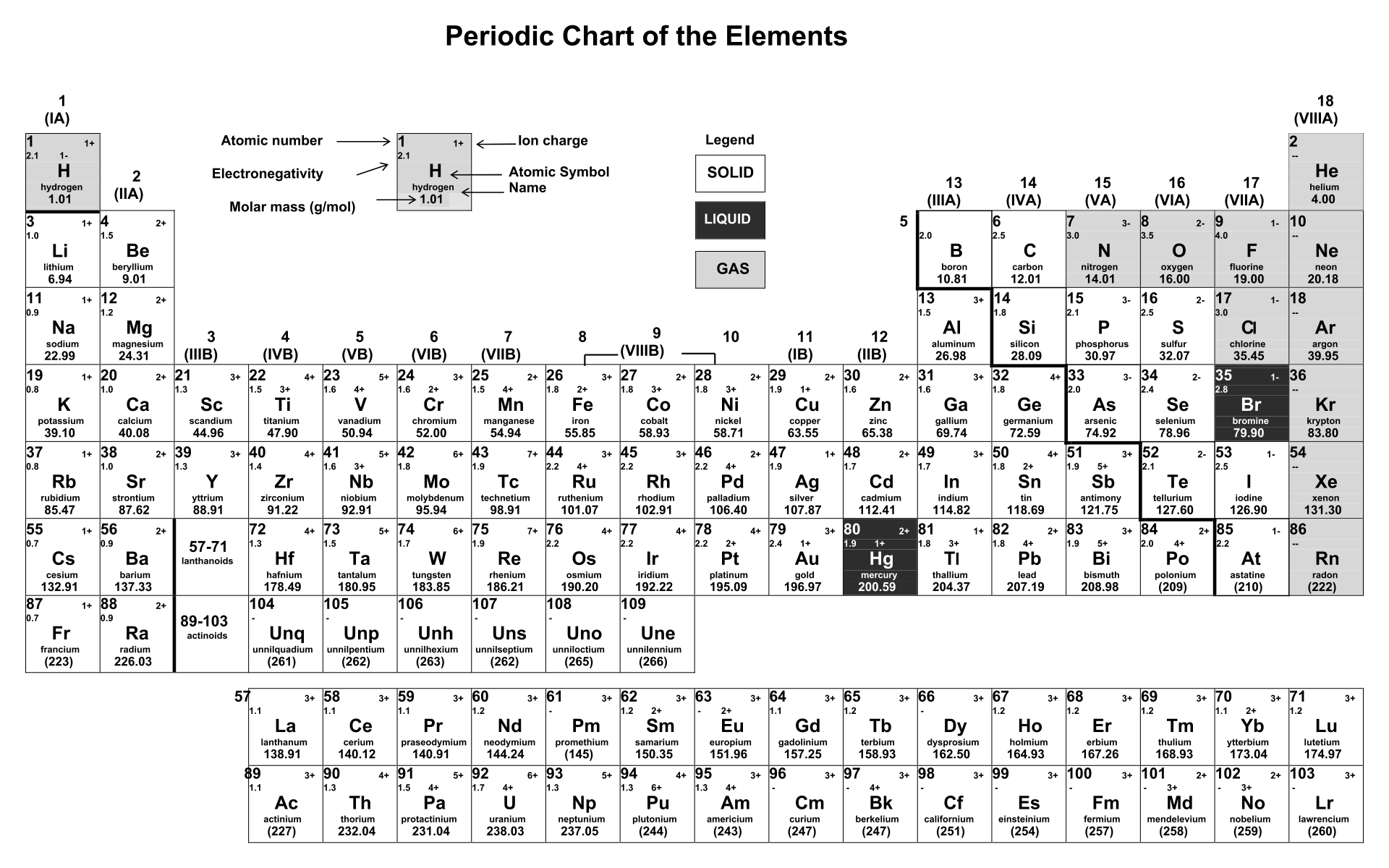
A printable Periodic Table table of ions offers a quick and accessible reference for students and professionals working in chemistry or related fields. It helps you understand the relationships between different elements, their charges, and their roles in chemical reactions.
You can easily identify trends in ionization energies, predict the formation of certain compounds, and enhance your grasp of chemical processes by having this visual aid at your disposal. Ideal for exam preparation, homework help, or on-the-spot queries, it simplifies complex information into an easy-to-follow format, boosting your efficiency and comprehension in studies or work tasks.
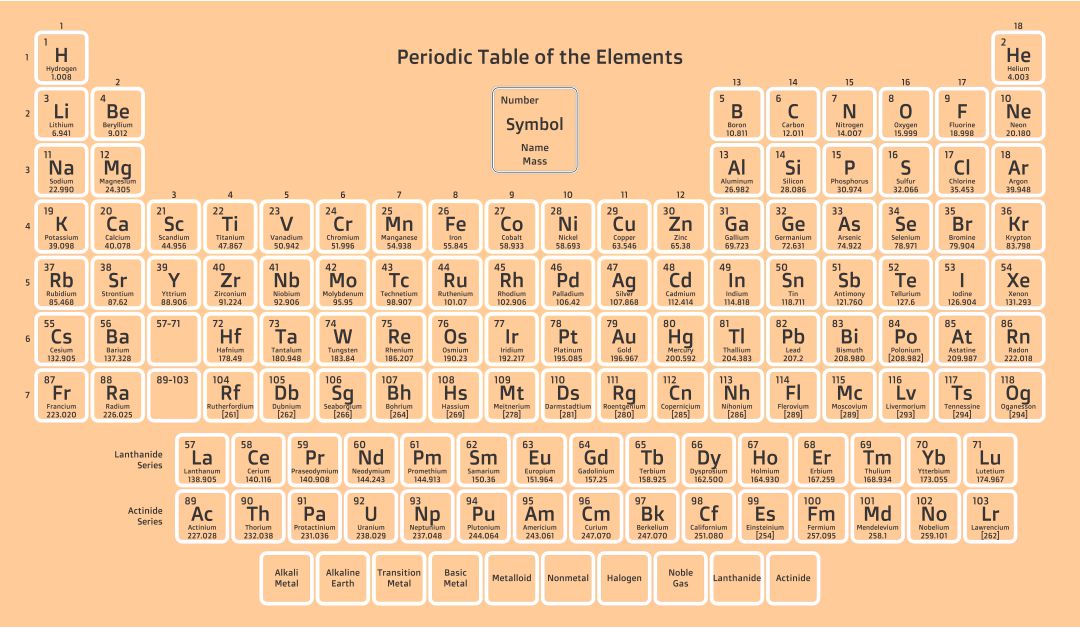
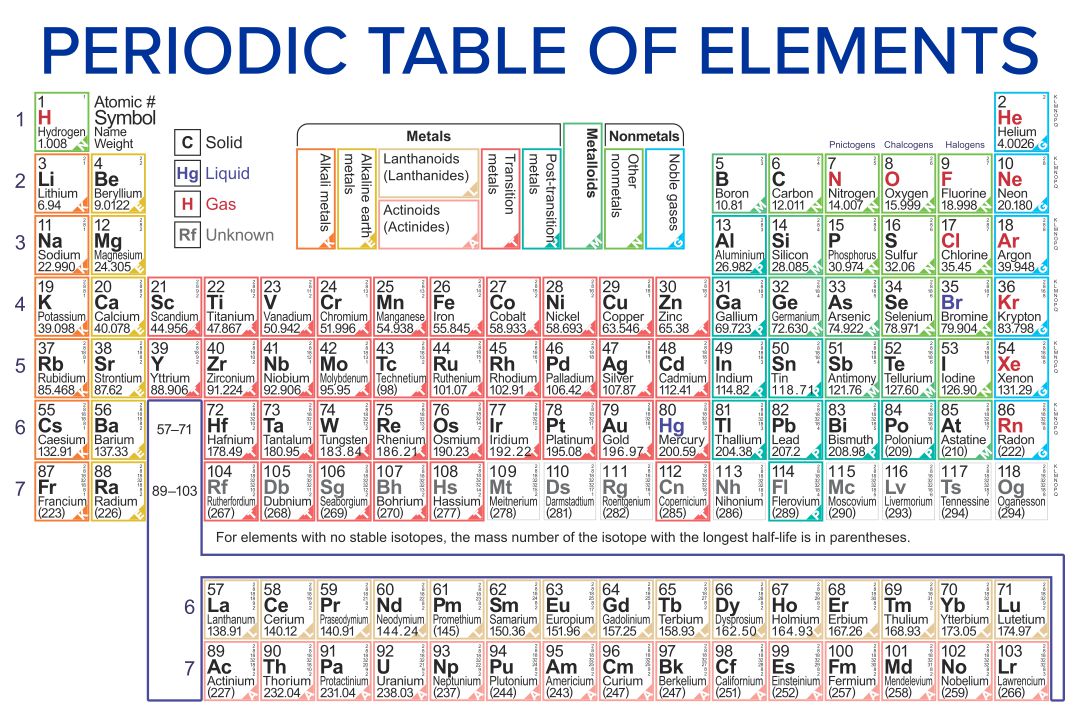
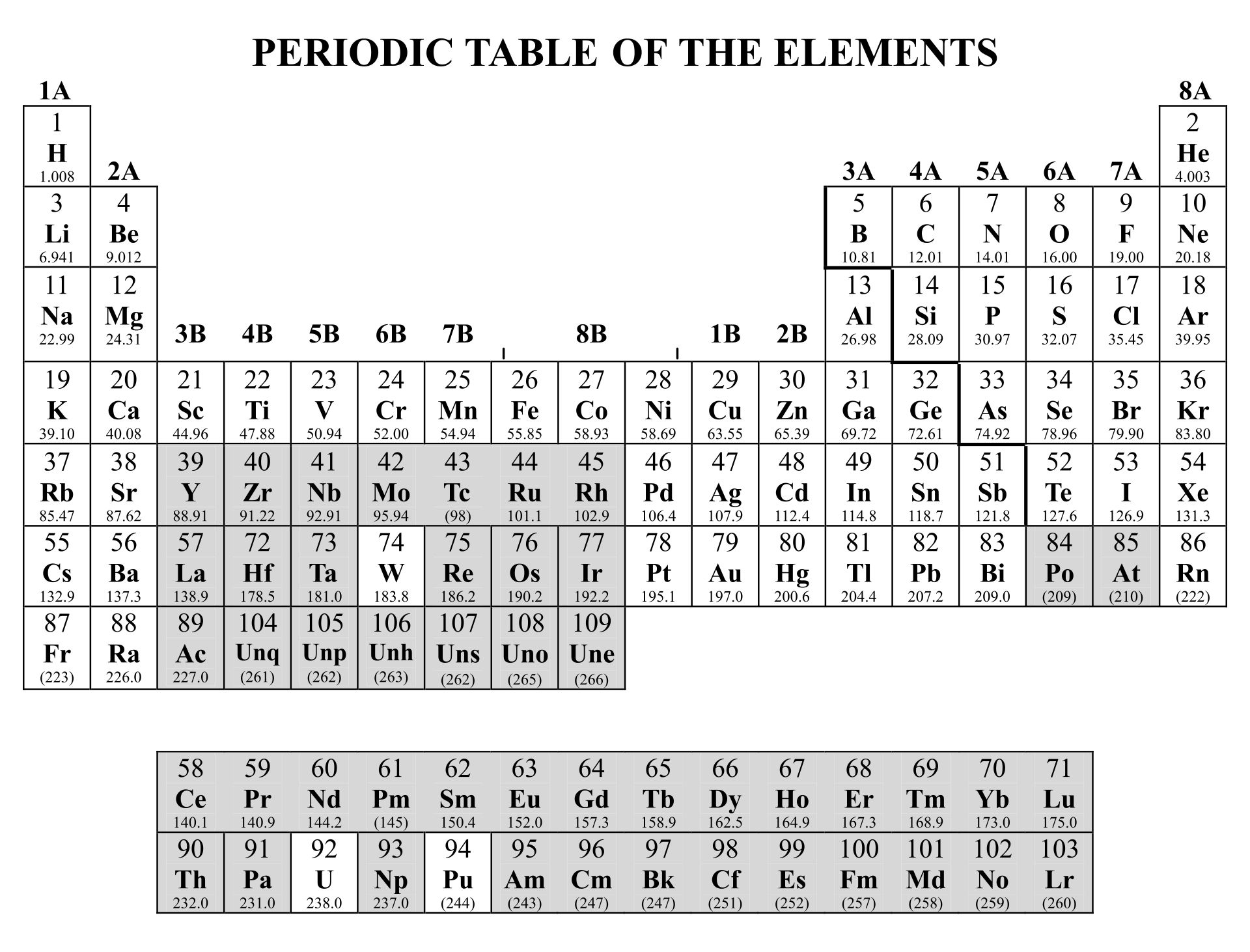
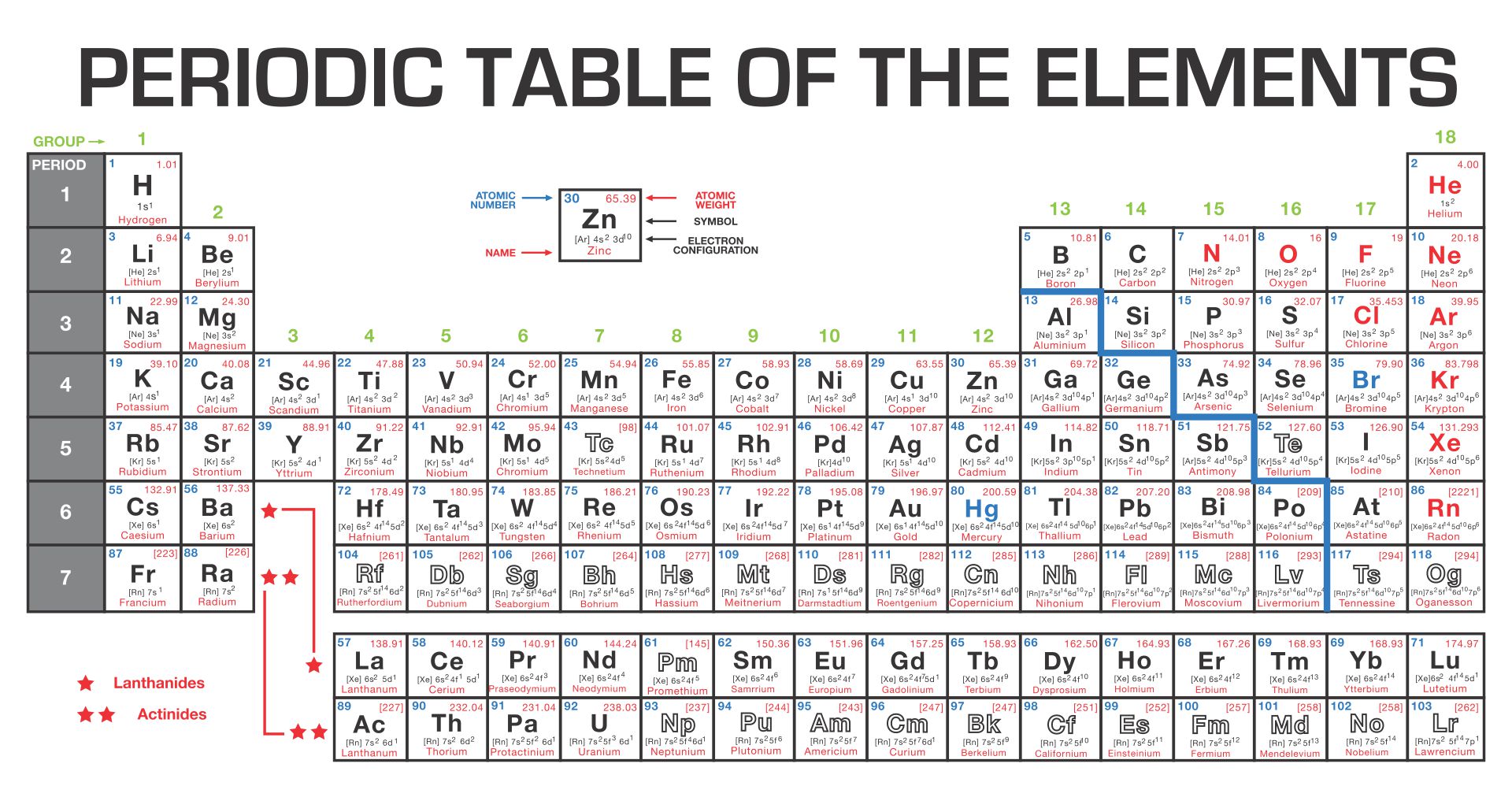

Understanding the fundamental differences between covalent and ionic bonds is crucial for mastering chemistry. A periodic table highlighting these bonds helps you visualize how elements interact, aiding in predicting compound properties and chemical reactions. This can enhance your grasp of molecular structures and bonding mechanisms.
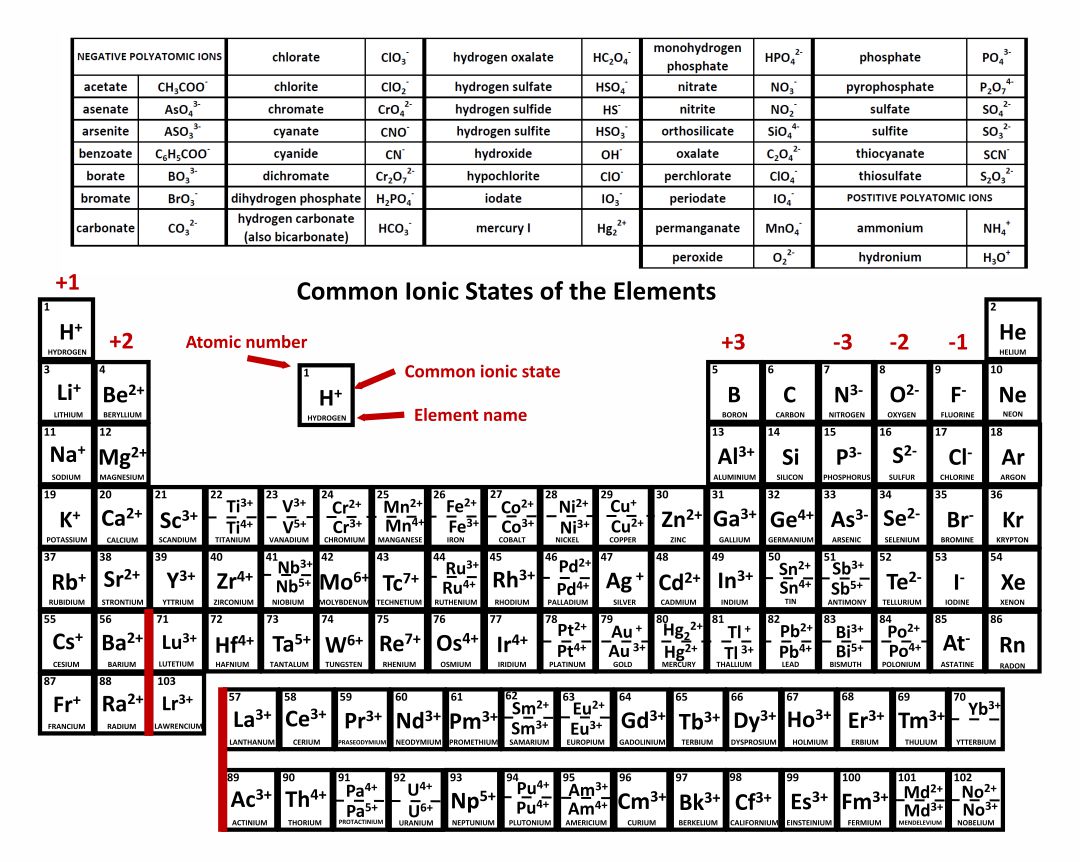
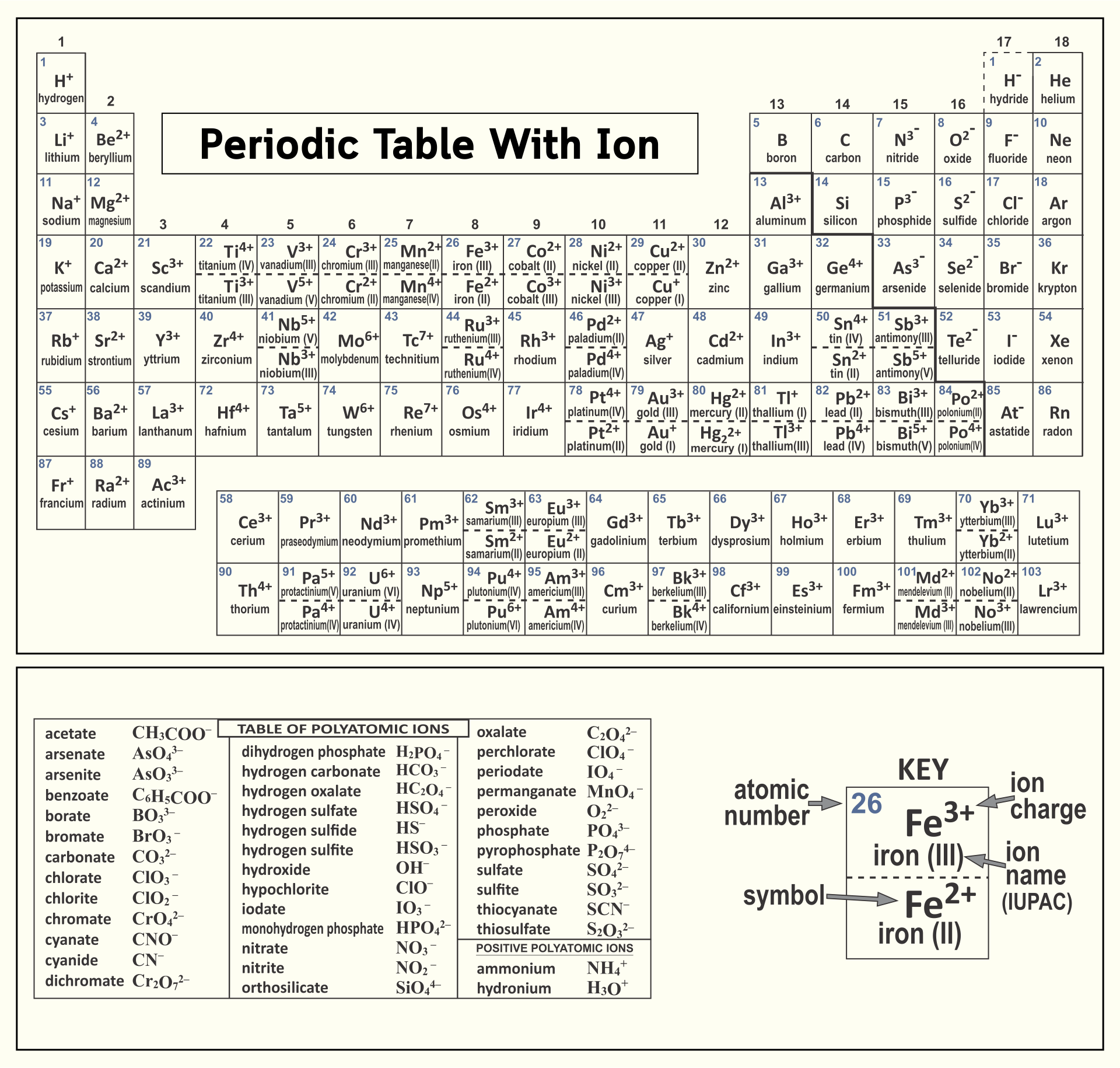
A periodic table featuring ions offers insights into how elements form ions, including their charges and sizes. This is essential for your studies in chemistry, particularly in understanding chemical formulas, reactions, and balancing equations. It's a practical tool for visual learners aiming to solidify their grasp of ionic properties in elements.
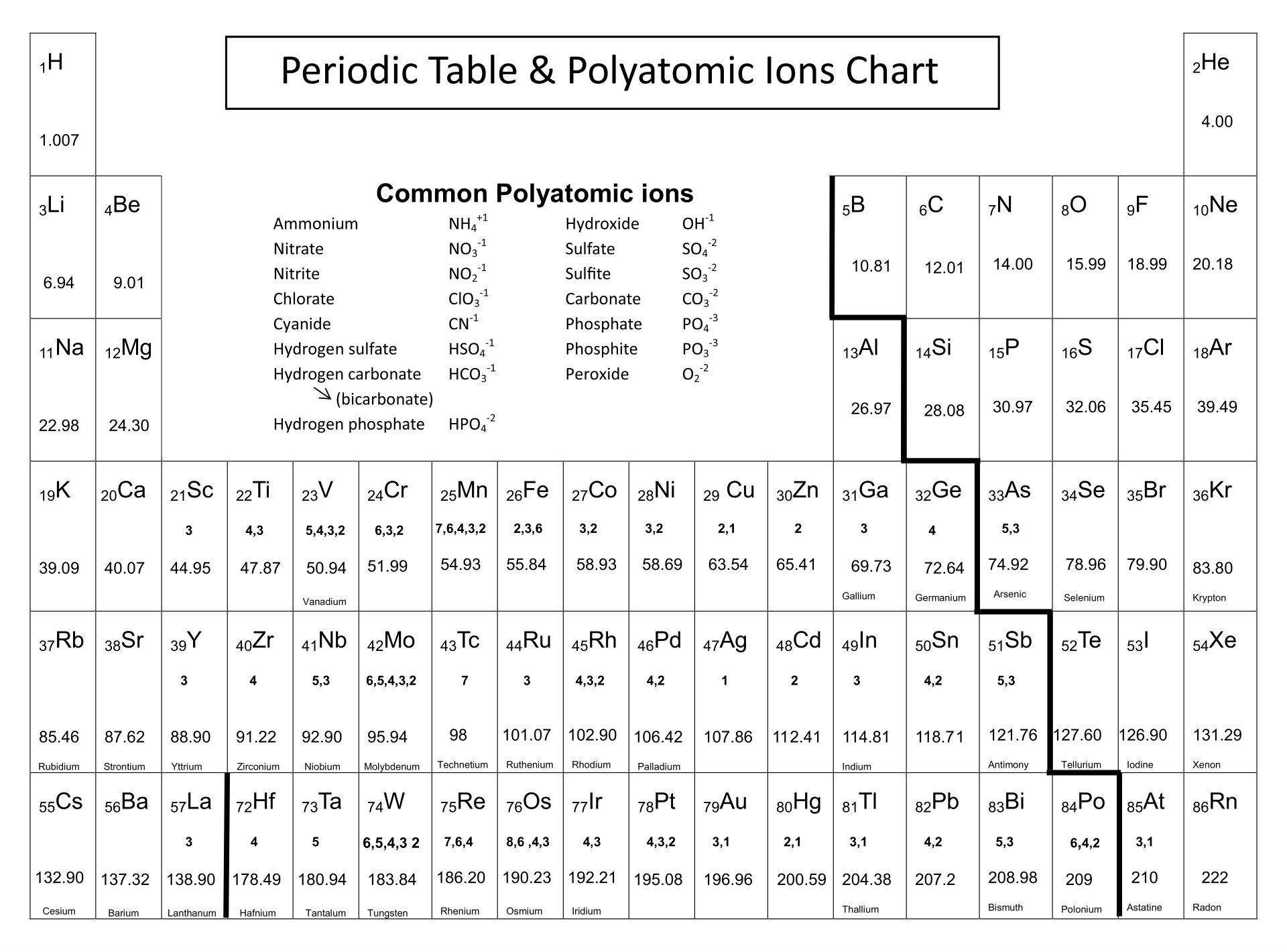
Having a printed version of the periodic table featuring polyatomic ions at your disposal can significantly improve your study and reference efficiency. It's particularly helpful for quick reviews, memorization of polyatomic ions, and their use in chemical equations. This simplifies complex topics in chemistry, making it easier to understand and apply concepts related to polyatomic ions.
Have something to tell us?
Recent Comments
The printable periodic table of ions allows learners to visualize and better understand the different charge states of ions, aiding in the study and comprehension of chemical reactions.
The printable periodic table of ions is a useful tool for students and researchers, providing a clear and easily accessible reference for understanding and studying the various ions and their properties in a concise format.
The printable periodic table of ions is a useful resource that provides a clear and organized overview of different ions, aiding in memorization and understanding of their properties for chemistry students and professionals.