A printable version of the periodic table can greatly aid your study of chemistry. It provides quick access to key element information, including atomic numbers, symbols, and weights. Having such a resource can enhance your understanding and study efficiency.
For chemistry teachers, a printable periodic table offers a clear and convenient way to explain complex concepts. It's a practical tool that simplifies teaching and enriches students' learning experience.
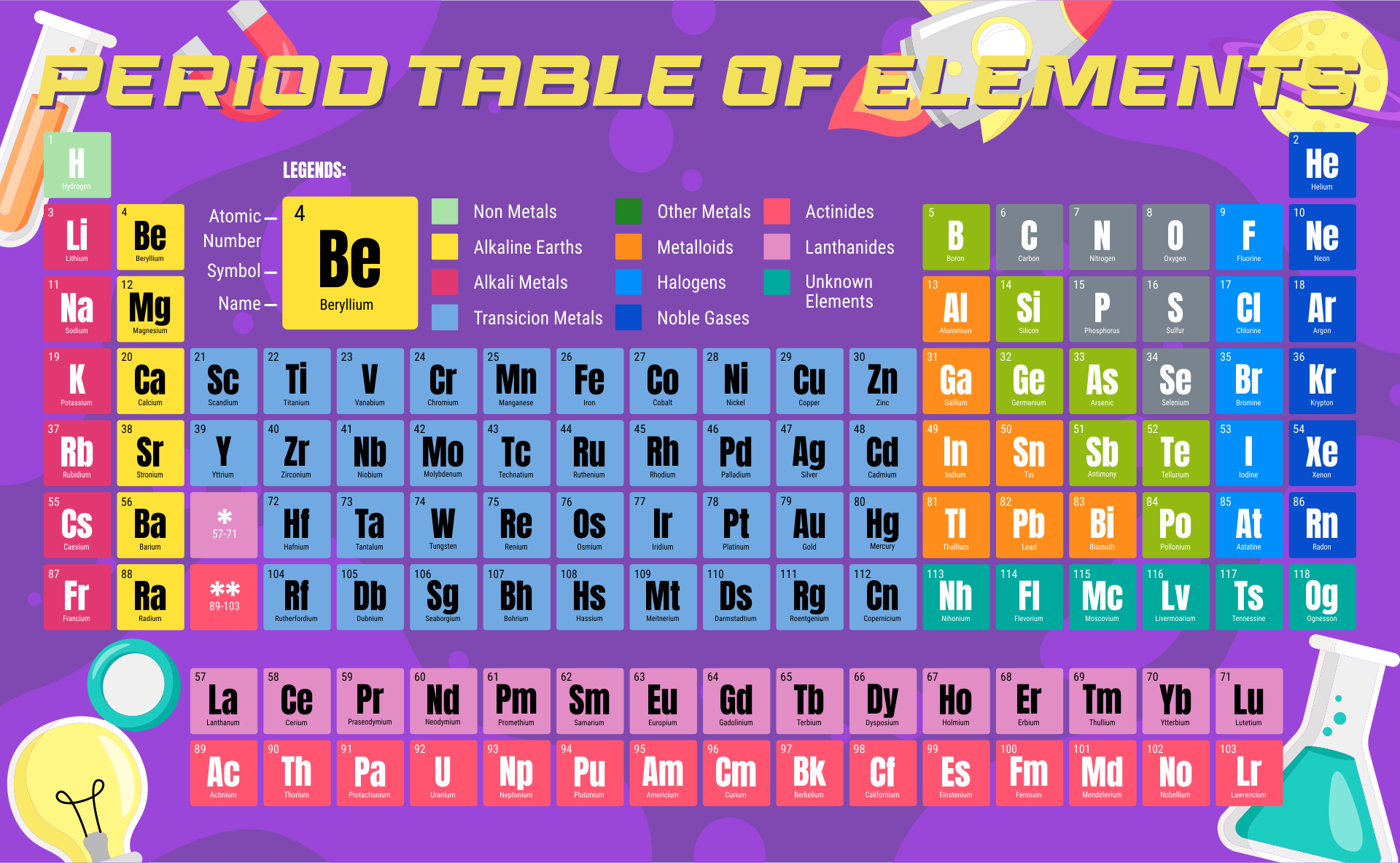
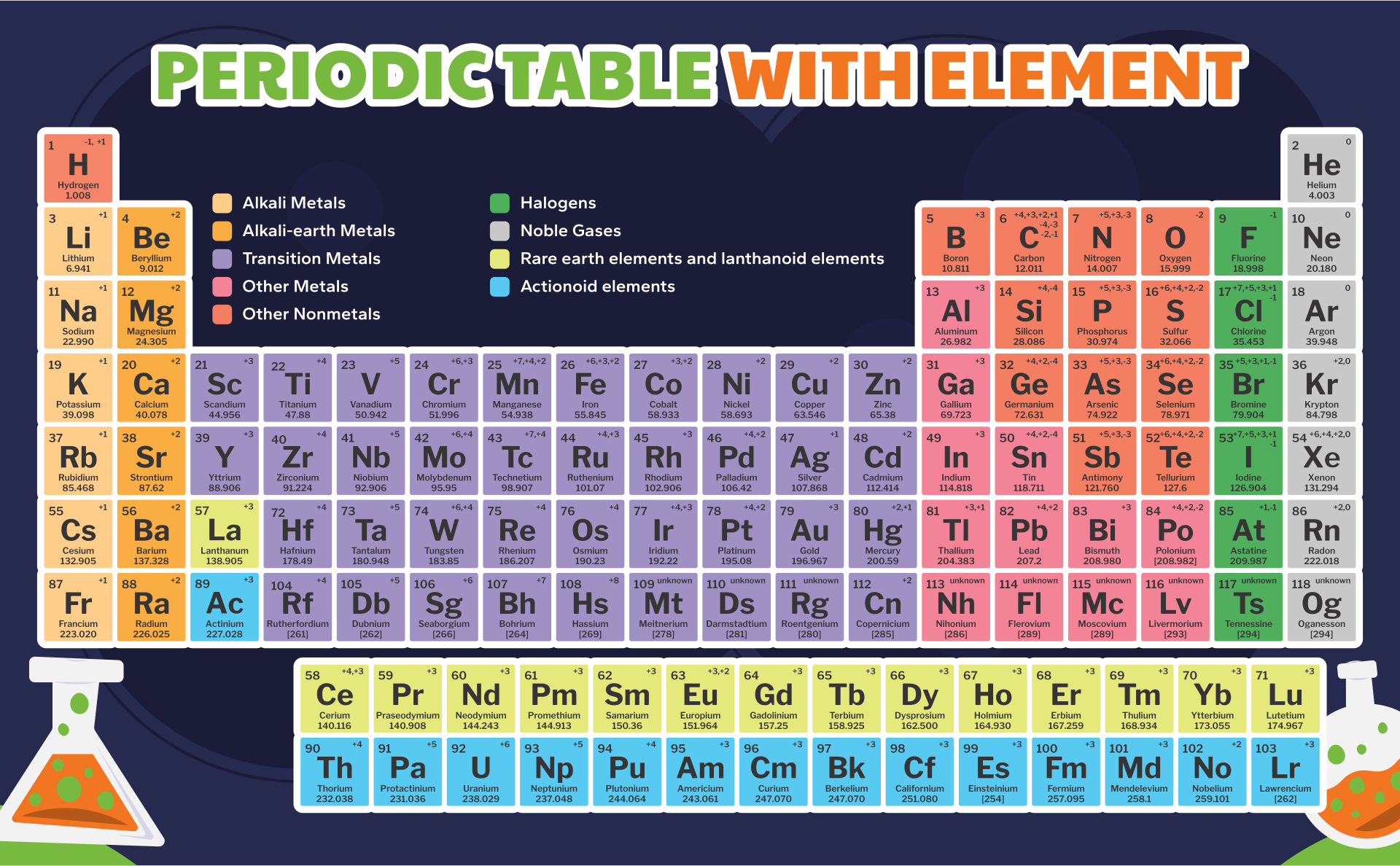
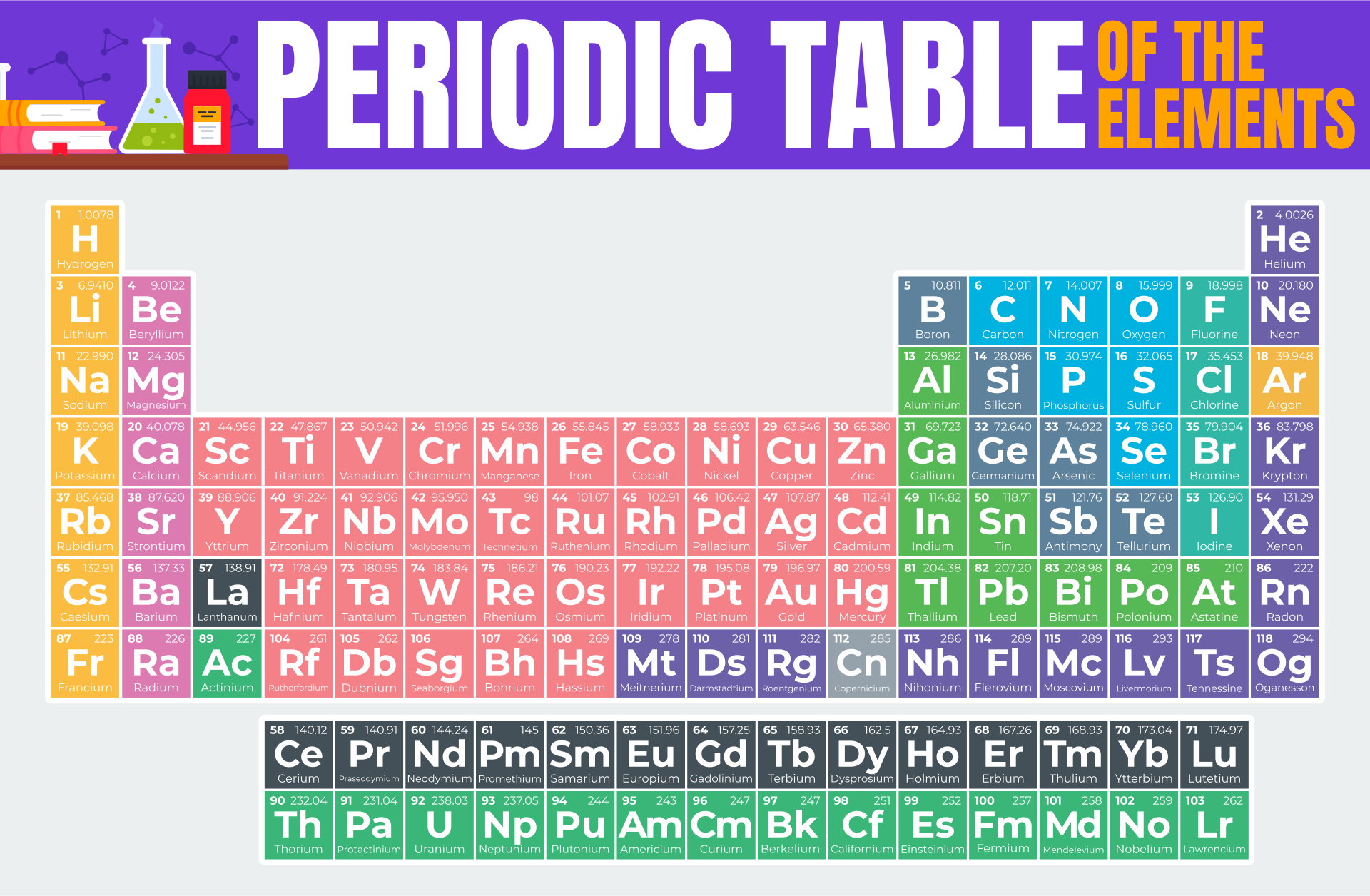
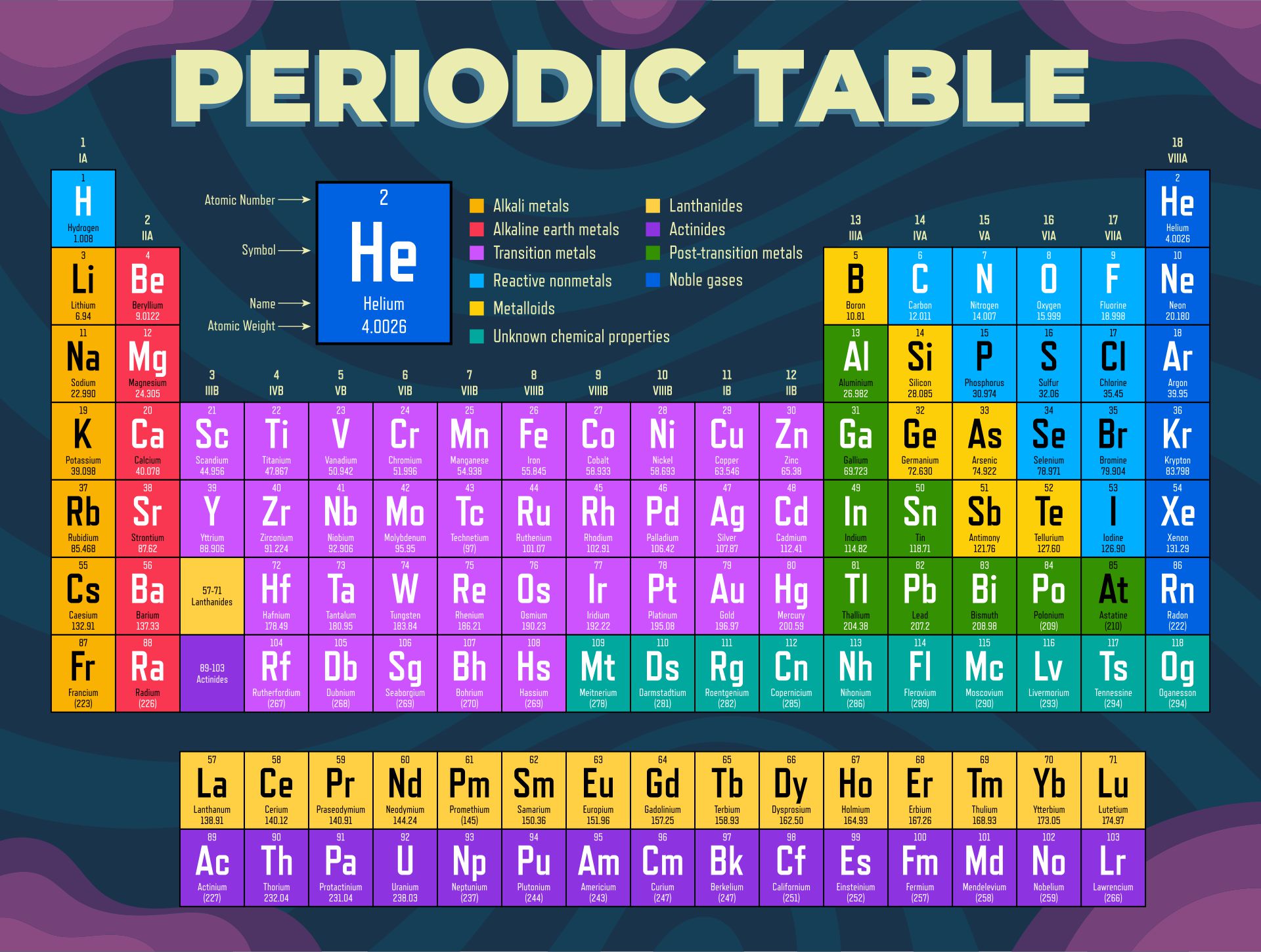
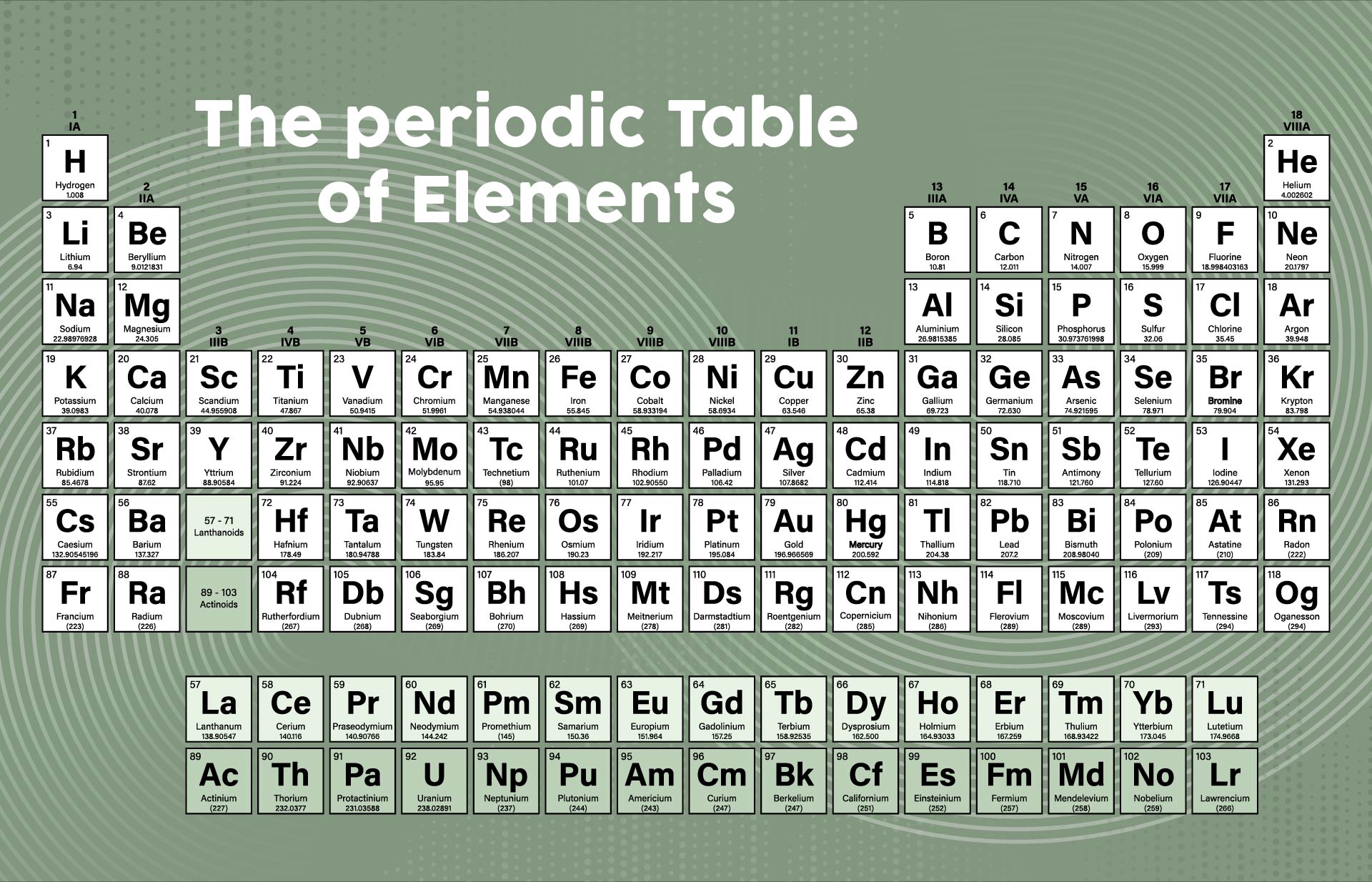
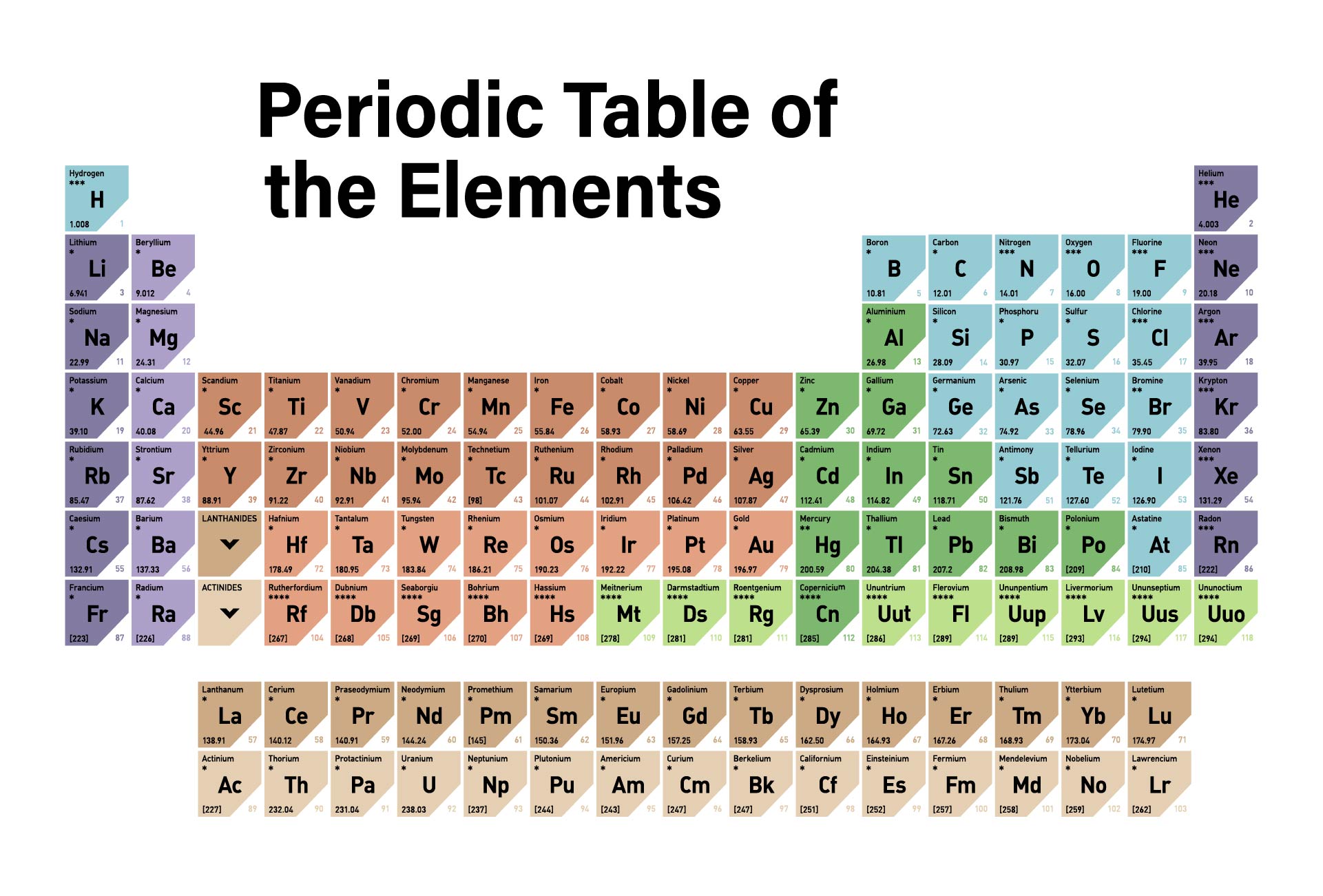
A periodic table printable version refers to a condensed and simplified version of the periodic table that can be easily printed and used for reference. It contains all the elements arranged in rows and columns based on their atomic number, symbols, and atomic weight. Having a printable version can be useful for studying and learning about the elements, their properties, and their relationships in a compact and accessible format.
Have something to tell us?
Recent Comments
The periodic table printable version simplifies the organization of elements and provides a convenient reference tool for students and professionals studying chemistry.
Thanks for providing the Periodic Table Printable Version! It's a helpful resource for students and professionals alike. Appreciate the simplicity and clarity in its design.
The printable version of the periodic table allows users to easily access and study the elements' characteristics, enhancing their understanding and knowledge of chemistry.