Engaging your preschooler with printable Easter Activities activities helps develop their creativity, fine motor skills, and understanding of the holiday. You'll find a variety of options such as coloring pages, simple puzzles, and cut-and-paste crafts, all designed to be fun and educational.
These activities can keep your child entertained while reinforcing important concepts like counting, pattern recognition, and following instructions. Perfect for a quiet afternoon or as a part of your Easter celebration, they offer a hands-on learning experience that your preschooler will love.

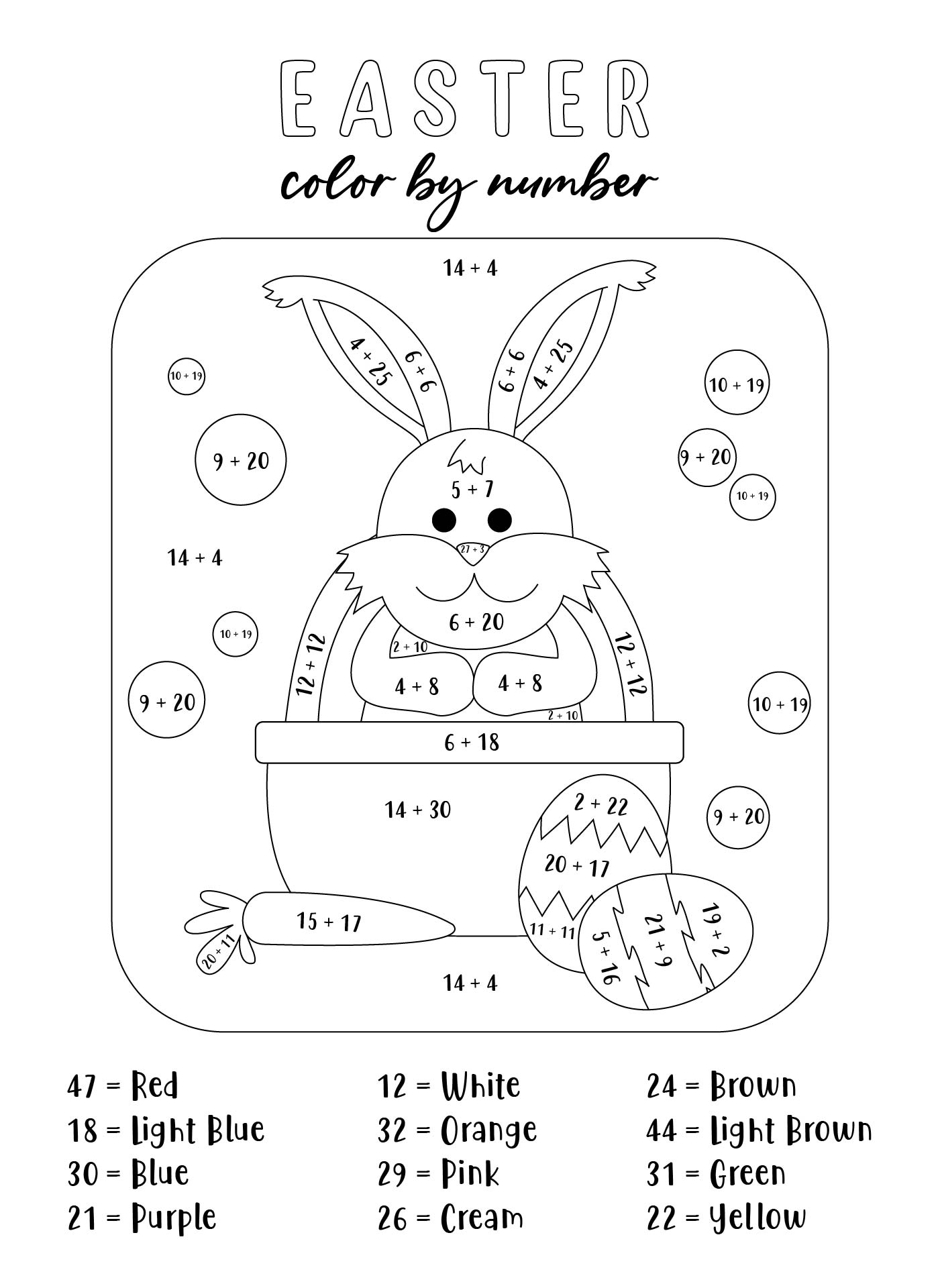
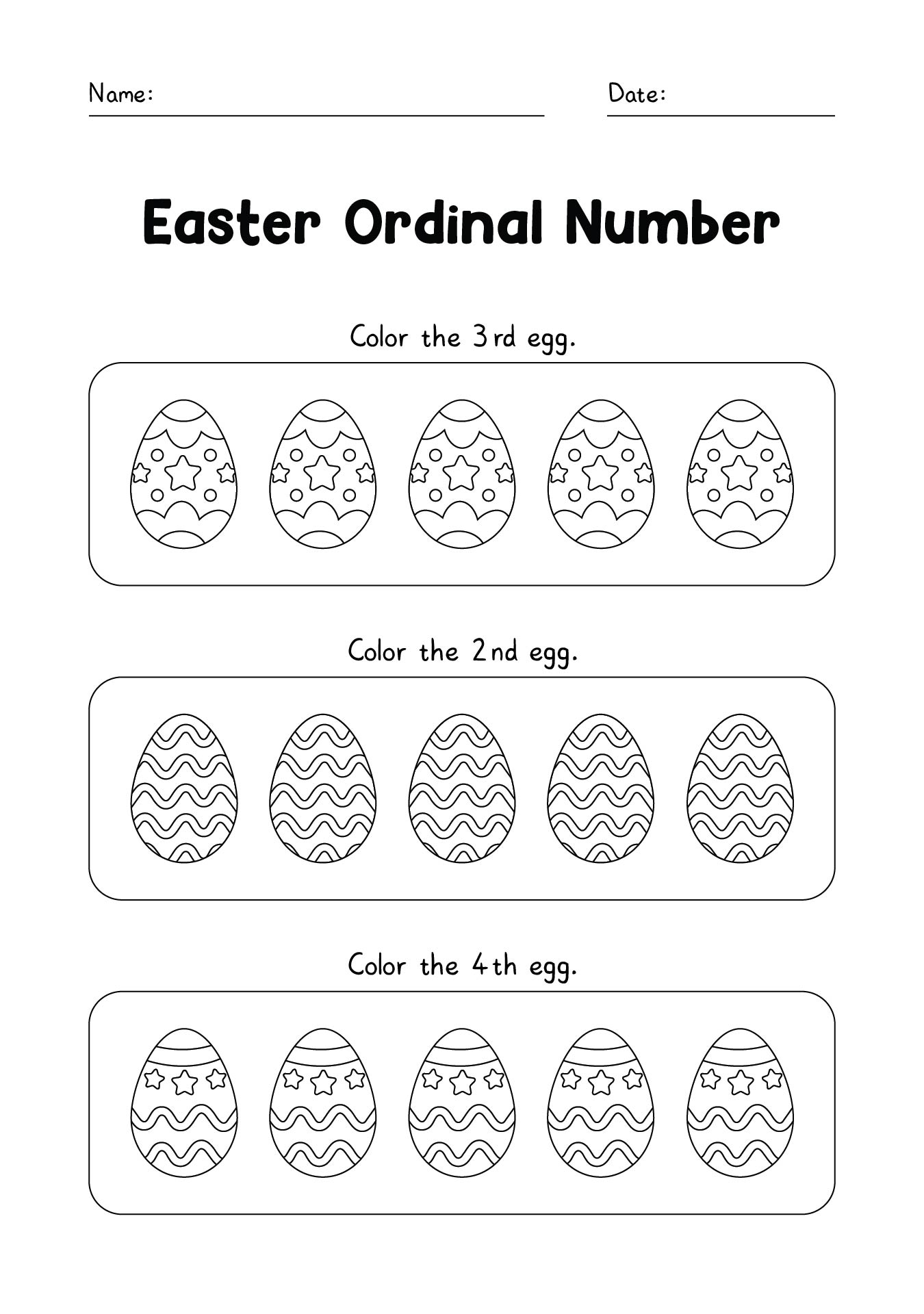
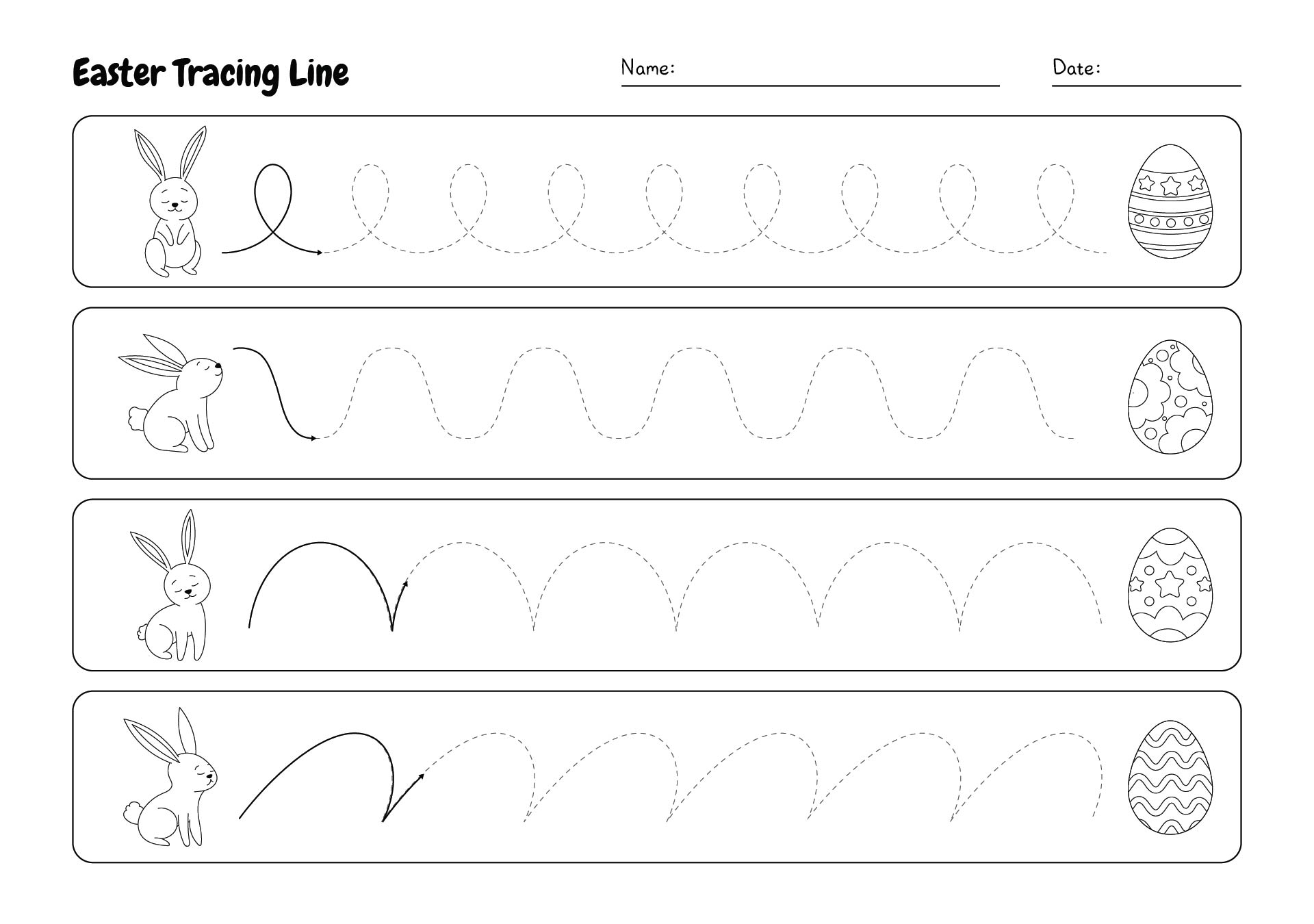
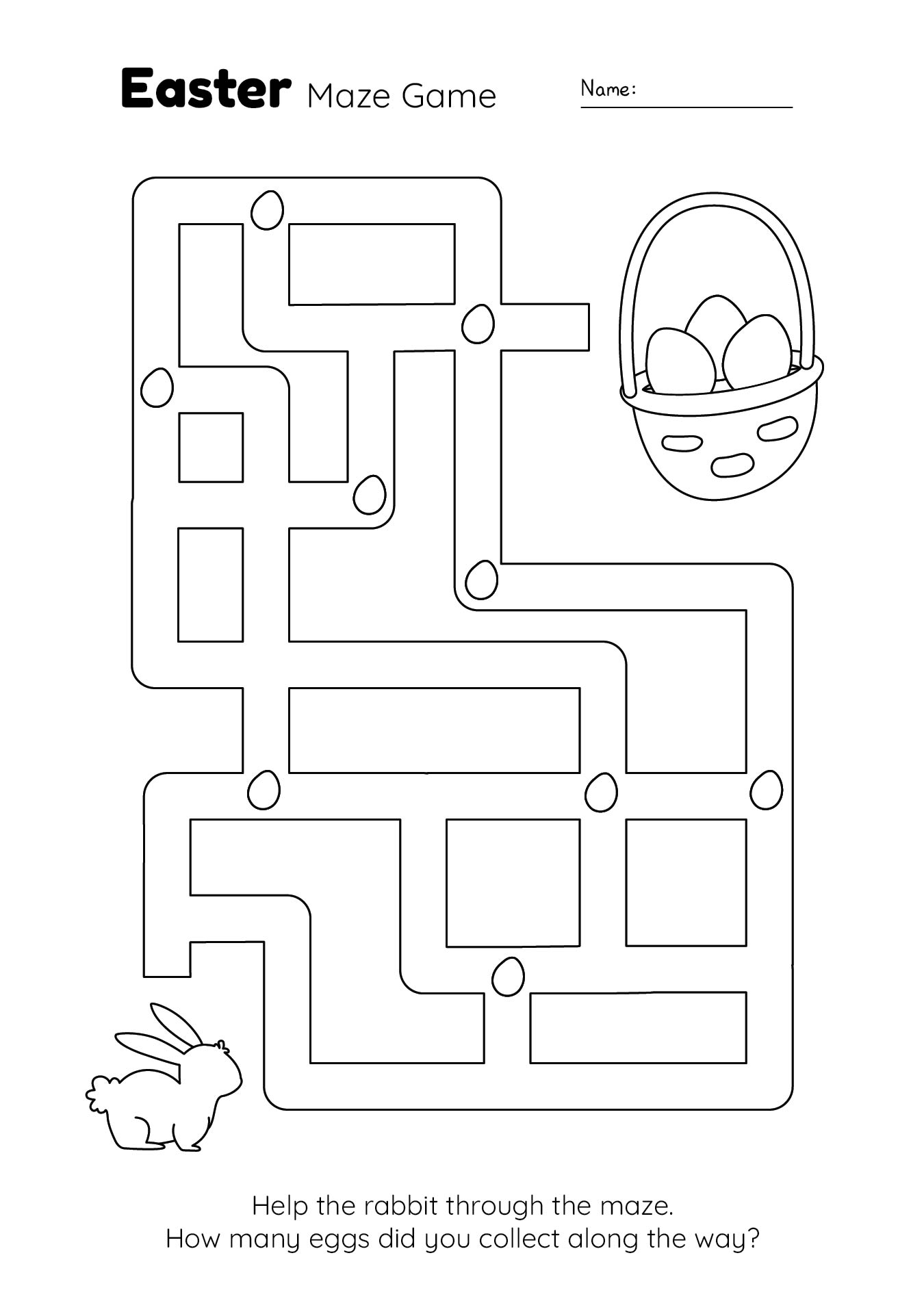

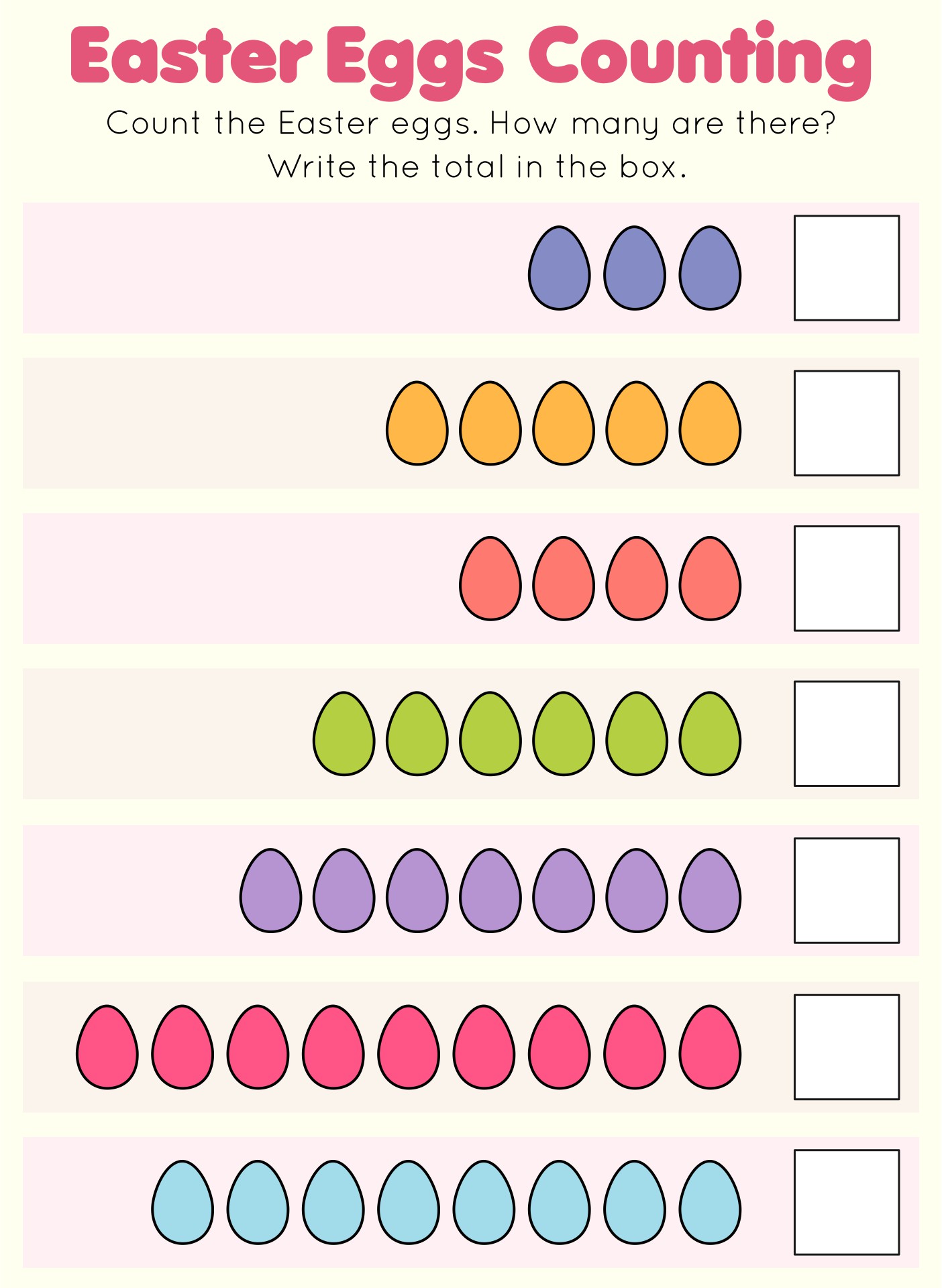


This activity offers a fun and engaging way for your young ones to practice their counting skills during the Easter season. By counting colorful Easter eggs, children can improve their number recognition and counting abilities, making it an enjoyable learning experience.
Easter Egg Activity Pages provide a variety of puzzles, coloring pages, and games centered around the Easter theme. These printables are designed to entertain and educate, helping children develop fine motor skills, problem-solving abilities, and creativity.
These worksheets are designed for busy parents and teachers who need quick, ready-to-use educational resources for preschoolers. Covering various skills such as alphabet recognition, basic math, and shape identification, they support early learning in a fun and accessible way.
Have something to tell us?
Recent Comments
Printable easter activities for preschoolers provide a convenient and engaging way to cultivate creativity and fine motor skills in young children, offering a wide range of enjoyable and educational exercises that can be easily accessed and completed at home.
Printable Easter activities for preschoolers are a convenient and educational way to engage children in fun and creative exercises, providing them with an opportunity to develop crucial skills while having a great time.
I'm so grateful for this printable resource! It's perfect for keeping my preschooler engaged and excited during Easter season. Thank you for making learning and celebrating so much fun!